Objective: The objective of this retrospective study was to assess the effectiveness and safety of flumatinib in the treatment of chronic phase chronic myeloid leukemia (CML) in real-world settings.
Methods: A total of 61 patients who received flumatinib as first-line therapy were compared with 92 patients who received first-line imatinib therapy. Furthermore, the 58 patients who received flumatinib as post-line therapy were divided into two groups based on the timing of switching to flumatinib: an early conversion group (n=18) and a late conversion group (n=40). The molecular responses and outcomes were assessed at multiple time points (3, 6, 9, and 12 months) during the treatment of flumatinib, along with the observation of adverse reactions.
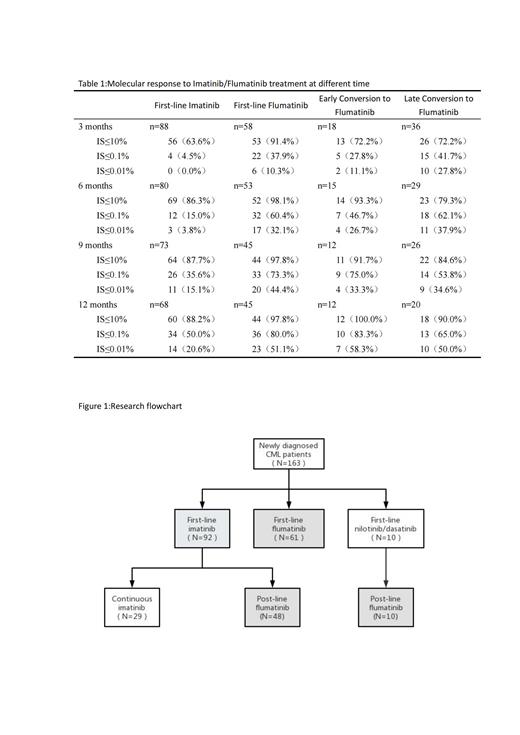
Results: In comparison to imatinib, the use of flumatinib as a first-line therapy demonstrated statistically significant increases in early molecular response (EMR), major molecular response (MMR), and deep molecular response (DMR) at various milestones (p<0.05). Additionally, the incidence of adverse events was lower in the flumatinib group, as evidenced by higher event-free survival (EFS) rates (83.6% vs. 39.1%, P=0.01) and a lower medication switching rate (90.2% vs. 31.5%, P<0.001). However, there were no significant differences observed in overall survival (OS) and progression-free survival (PFS) rates between the two groups (P>0.05). In the context of post-line therapy, a significant proportion of patients (72.2%) achieved EMR at 3 months, while 71.9% achieved MMR at 12 months when treated with flumatinib. Notably, the early conversion group exhibited higher rates of molecular response and demonstrated superior OS, PFS, and EFS at the 12-month mark compared to the late conversion group (P>0.05). Adverse events (AEs) commonly associated with flumatinib encompassed limb discomfort, gastrointestinal reactions, and elevated transaminase levels, with thrombocytopenia being the most frequently observed hematologic AE.
Conclusion: Flumatinib exhibited notable efficacy and safety as a therapeutic option for patients with chronic phase CML, whether utilized as a first-line or post-line treatment. In comparison to imatinib, initial administration of flumatinib resulted in more profound and expeditious molecular responses. The utilization of flumatinib as a post-line therapeutic approach during the early stage of treatment exhibits a favorable influence on patient prognosis. However, no statistically significant disparity was noted when compared to the group receiving switch therapy during the late stage.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal